
Chemistry, 26.04.2021 07:00 gabriellestaleyga16
If 57.5 mol of an ideal gas is at 4.97 atm at 69.80 ∘C, what is the volume of the gas?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 23.06.2019 03:00
Can someone me out on this question for my national 5 chemistry homework
Answers: 1
You know the right answer?
If 57.5 mol of an ideal gas is at 4.97 atm at 69.80 ∘C, what is the volume of the gas?...
Questions


Social Studies, 12.12.2020 15:50

World Languages, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50





Mathematics, 12.12.2020 15:50

Biology, 12.12.2020 15:50


English, 12.12.2020 15:50


History, 12.12.2020 15:50



Law, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50

Social Studies, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50

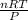
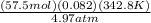 = 325 L
= 325 L

