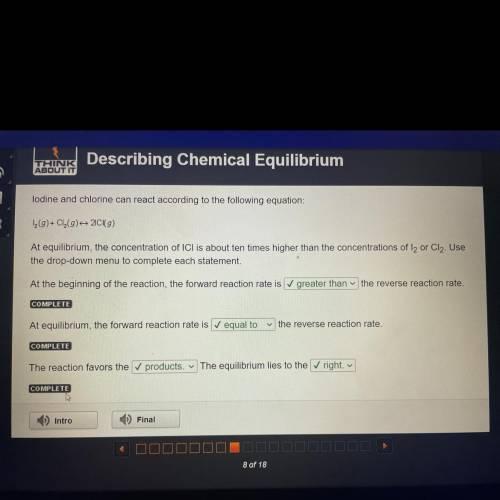
Lodine and chlorine can react according to the following equation:
1 (9)+ Cl(9) 20 (9)
At eq...

Chemistry, 26.04.2021 07:40 kinglightskin2k
Lodine and chlorine can react according to the following equation:
1 (9)+ Cl(9) 20 (9)
At equilibrium, the concentration of ICI is about ten times higher than the concentrations of l2 or Cl2. Use
the drop-down menu to complete each statement.
At the beginning of the reaction, the forward reaction rate is greater than the reverse reaction rate.
COMPLETE
At equilibrium, the forward reaction rate is equal to
the reverse reaction rate.
COMPLETE
The reaction favors the ✓ products. The equilibrium lies to the right.
COMPLETE

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3

You know the right answer?
Questions

History, 09.07.2019 14:40



Arts, 09.07.2019 14:40

Mathematics, 09.07.2019 14:40


Physics, 09.07.2019 14:40






Physics, 09.07.2019 14:50




Mathematics, 09.07.2019 14:50

History, 09.07.2019 14:50

Mathematics, 09.07.2019 14:50

Chemistry, 09.07.2019 14:50



