
Chemistry, 26.04.2021 14:00 oliviaprejean18
A 9.02-g sample of liquid water at 40.3 °C is heated by the addition of 203.93 J of energy. The final temperature of the water is °C. The specific heat capacity of liquid water is 4.184 J/gK.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
A 9.02-g sample of liquid water at 40.3 °C is heated by the addition of 203.93 J of energy. The fina...
Questions


Mathematics, 12.02.2022 22:50


English, 12.02.2022 22:50






English, 12.02.2022 22:50

Mathematics, 12.02.2022 22:50


English, 12.02.2022 22:50







Physics, 12.02.2022 22:50

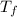 -
- 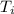
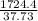 =
= 


