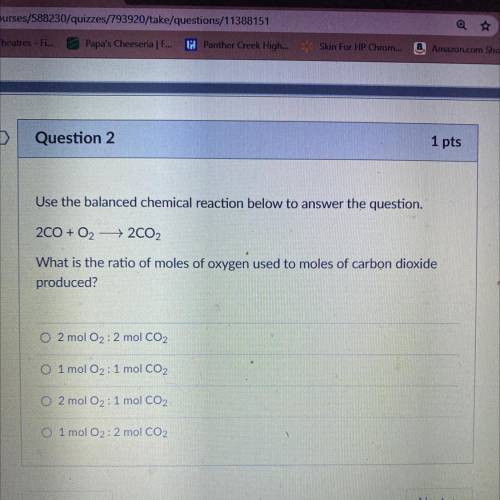
Question 2
Use the balanced chemical reaction below to answer the question.
200 + O2 + 2CO2<...


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 23.06.2019 07:20
Which statement explains which component is likely to be more powerful in explaining a scientific phenomenon? a) component c, because a theory is often passed on possibility and not certainty b) component d, because a hypothesis is often based on possibility not certainty c) component c, because the ability to explain several occurrences in the natural world is a characteristic of a hypothesis d) component d, because the ability to explain several occurrences in the natural world is a characteristic of a theory
Answers: 3
You know the right answer?
Questions

Chemistry, 19.12.2019 16:31

Biology, 19.12.2019 16:31

Physics, 19.12.2019 16:31




Mathematics, 19.12.2019 16:31




Mathematics, 19.12.2019 16:31

Biology, 19.12.2019 16:31








Biology, 19.12.2019 16:31