Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1


Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1
You know the right answer?
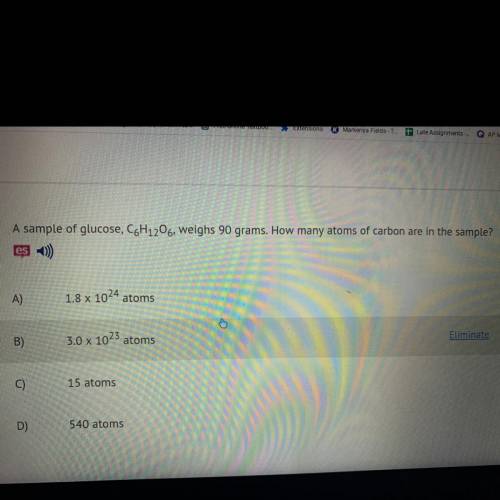
A sample of glucose, C6H12O6, weighs 90 grams. How many atoms of carbon are in the sample?
A)
Questions

World Languages, 16.04.2021 01:00


Advanced Placement (AP), 16.04.2021 01:00


History, 16.04.2021 01:00

Mathematics, 16.04.2021 01:00

Mathematics, 16.04.2021 01:00

History, 16.04.2021 01:00

Mathematics, 16.04.2021 01:00



Law, 16.04.2021 01:00






English, 16.04.2021 01:00

English, 16.04.2021 01:00