
Chemistry, 26.04.2021 21:10 tamikagoss22
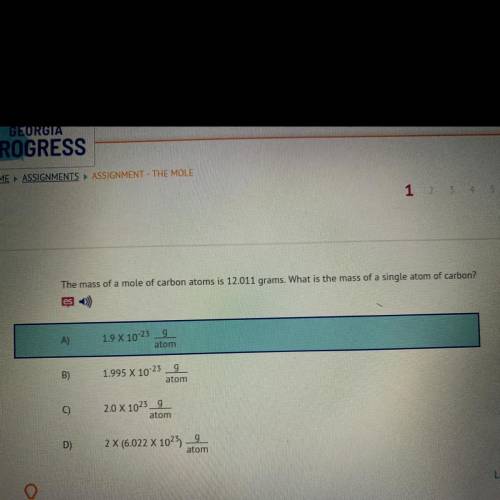
The mass of a mole of carbon atoms is 12.011 grams. What is the mass of a single atom of carbon?
A)9
1.9 X 10-23
atom
B)
g
1.995 X 10-23
atom
C)
2.0 X 1023_9
atom
D)
2 X (6.022 X 1025)
9
atom
Law of Conserv
Y

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
You know the right answer?
The mass of a mole of carbon atoms is 12.011 grams. What is the mass of a single atom of carbon?
A...
Questions

History, 15.10.2019 12:30




Biology, 15.10.2019 12:30

Chemistry, 15.10.2019 12:30


Mathematics, 15.10.2019 12:30


History, 15.10.2019 12:30




History, 15.10.2019 12:30



English, 15.10.2019 12:30

History, 15.10.2019 12:30




