
Chemistry, 26.04.2021 22:00 monkeys450
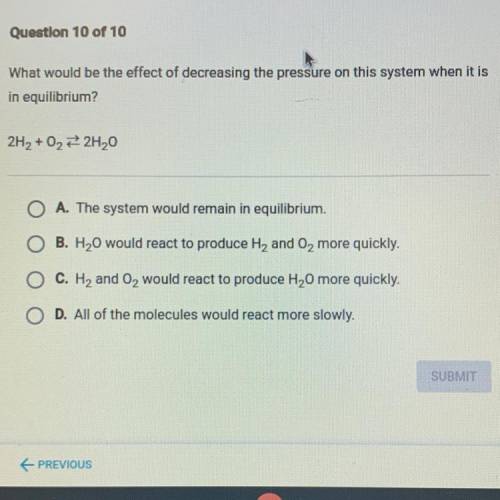
What would be the effect of decreasing the pressure on this system when it is
in equilibrium?
2H2 + O2 2H20
A. The system would remain in equilibrium.
B. H2O would react to produce H2 and O2 more quickly.
C. H2 and O2 would react to produce H20 more quickly.
D. All of the molecules would react more slowly.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1



You know the right answer?
What would be the effect of decreasing the pressure on this system when it is
in equilibrium?
Questions

Mathematics, 02.03.2020 15:18

English, 02.03.2020 15:18

Mathematics, 02.03.2020 15:18


World Languages, 02.03.2020 15:21


World Languages, 02.03.2020 15:22


Business, 02.03.2020 15:23

Computers and Technology, 02.03.2020 15:23

English, 02.03.2020 15:23

Mathematics, 02.03.2020 15:24

English, 02.03.2020 15:24

Mathematics, 02.03.2020 15:25

Physics, 02.03.2020 15:25


Mathematics, 02.03.2020 15:26

World Languages, 02.03.2020 15:27




