
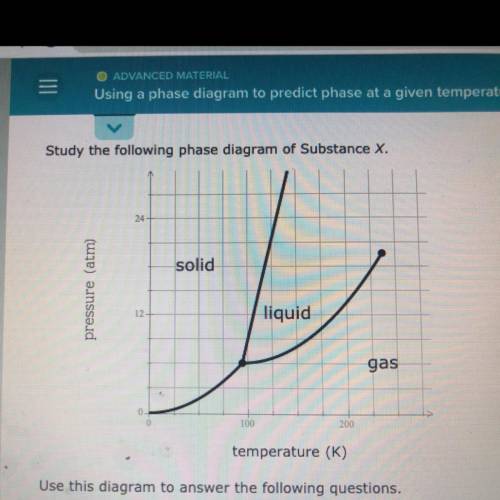
Use this diagram to answer the following questions.
Suppose a small sample of pure X is held at -48. °C and 10.7 atm.
What will be the state of the sample?
(choose one)
Suppose the temperature is held constant at -48. °C but the pressure
is increased by 3.2 atm. What will happen to the sample?
(choose one)
Suppose, on the other hand, the pressure is held constant at 10.7 atm
but the temperature is decreased by 80. °C. What will happen to the
sample?
(choose
one)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

You know the right answer?
Use this diagram to answer the following questions.
Suppose a small sample of pure X is held at -4...
Questions

Mathematics, 10.05.2021 16:40

Mathematics, 10.05.2021 16:40


Mathematics, 10.05.2021 16:40

Mathematics, 10.05.2021 16:40

Chemistry, 10.05.2021 16:40



English, 10.05.2021 16:40

History, 10.05.2021 16:40









Mathematics, 10.05.2021 16:40



