
Chemistry, 27.04.2021 01:00 oscard1627
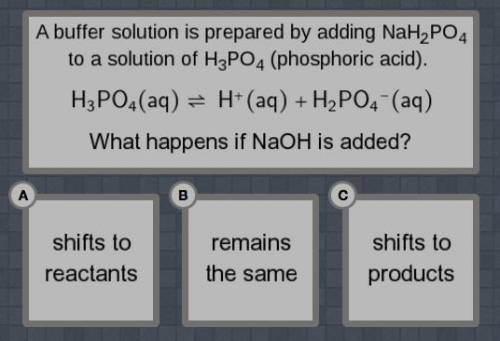
A buffer solution is prepared by adding NaH2PO4 to a solution of H3PO4 (phosphoric acid).
H3PO4(aq) = H+(aq)+H2PO4-(aq)
What happens if NaOH is added?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
A buffer solution is prepared by adding NaH2PO4 to a solution of H3PO4 (phosphoric acid).
H3PO4(aq...
Questions



Mathematics, 05.01.2021 16:40





Biology, 05.01.2021 16:40


Mathematics, 05.01.2021 16:40

History, 05.01.2021 16:40






Computers and Technology, 05.01.2021 16:40

Law, 05.01.2021 16:40


History, 05.01.2021 16:40



