The weak ionization constant (Ka)
for HNO2 is equal to:
...

Chemistry, 27.04.2021 04:10 inucornspineapple
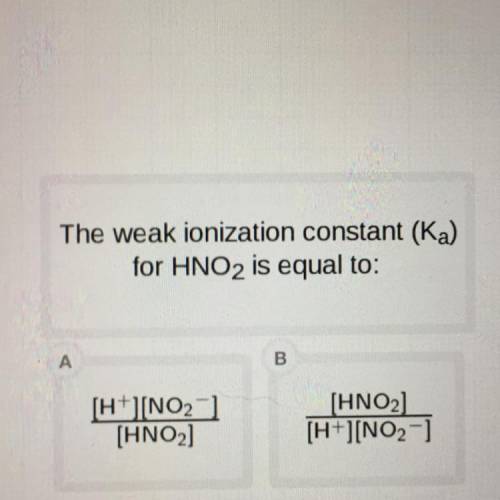
The weak ionization constant (Ka)
for HNO2 is equal to:

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

You know the right answer?
Questions





History, 19.11.2019 18:31


English, 19.11.2019 18:31















