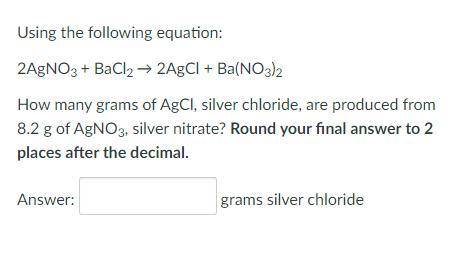
Using the following equation:
2AgNO3 + BaCl2 → 2AgCl + Ba(NO3)2
How many grams of AgCl...

Chemistry, 27.04.2021 18:20 darcyshay62871
Using the following equation:
2AgNO3 + BaCl2 → 2AgCl + Ba(NO3)2
How many grams of AgCl, silver chloride, are produced from 8.2 g of AgNO3, silver nitrate? Round your final answer to 2 places after the decimal.
_grams silver chloride

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 03:30
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1

Chemistry, 23.06.2019 07:30
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes no correct anwser get brainliest
Answers: 1

Chemistry, 23.06.2019 07:40
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1
You know the right answer?
Questions

Mathematics, 14.10.2019 10:10


Chemistry, 14.10.2019 10:10


Mathematics, 14.10.2019 10:10




Chemistry, 14.10.2019 10:10


Mathematics, 14.10.2019 10:10

Mathematics, 14.10.2019 10:10



Mathematics, 14.10.2019 10:10

History, 14.10.2019 10:10




Chemistry, 14.10.2019 10:10



