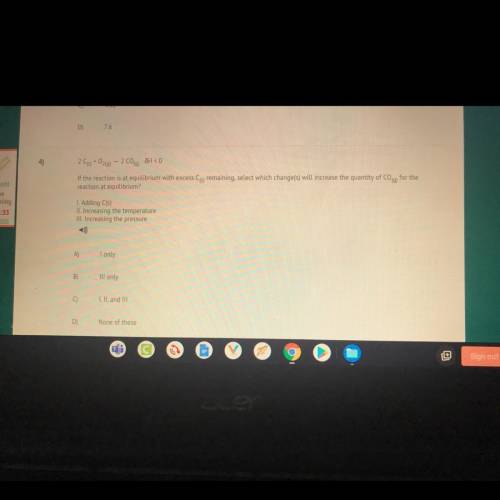
4)
209+0260) - 2 C00) JH < 0
If the reaction is at equilibrium with excess Cs remaining,...

Chemistry, 28.04.2021 02:50 mahagonylabeyta
4)
209+0260) - 2 C00) JH < 0
If the reaction is at equilibrium with excess Cs remaining, select which change(s) will increase the quantity of Coq) for the
reaction at equilibrium?
I. Adding C(s)
II. Increasing the temperature
III. Increasing the pressure

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3


Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
Questions










World Languages, 27.06.2020 15:01



English, 27.06.2020 15:01

English, 27.06.2020 15:01

Mathematics, 27.06.2020 15:01

Mathematics, 27.06.2020 15:01



Mathematics, 27.06.2020 15:01



