Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
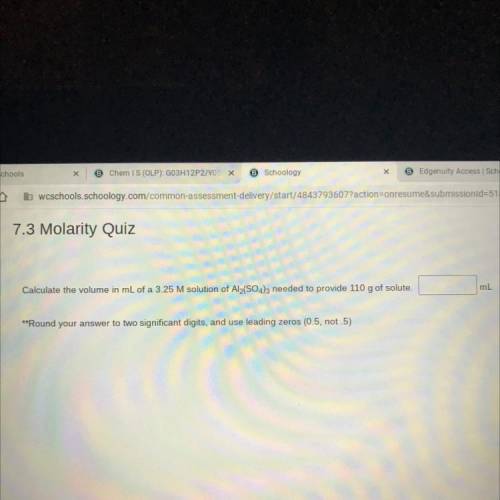
Calculate the volume in mL of a 3.25 M solution of Al2(SO4)3 needed to provide 110 g of solute.
Questions



Mathematics, 09.07.2019 01:00



English, 09.07.2019 01:00

English, 09.07.2019 01:00

Mathematics, 09.07.2019 01:00


Health, 09.07.2019 01:00


Mathematics, 09.07.2019 01:00

English, 09.07.2019 01:00



Mathematics, 09.07.2019 01:00


Mathematics, 09.07.2019 01:00