Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1
You know the right answer?
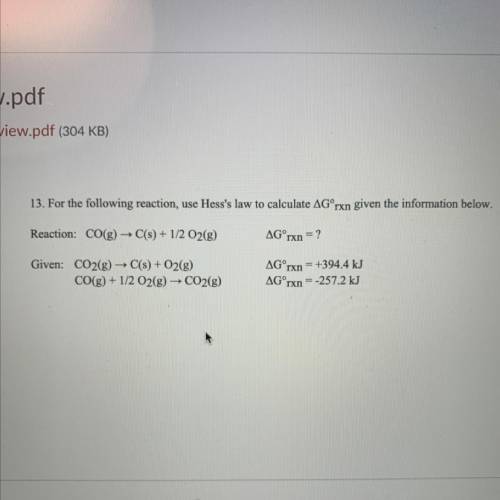
13. For the following reaction, use Hess's law to calculate AGʻrxn given the information below.
Re...
Questions


History, 26.09.2019 04:00



Social Studies, 26.09.2019 04:00



Social Studies, 26.09.2019 04:00

Mathematics, 26.09.2019 04:00

Physics, 26.09.2019 04:00

Health, 26.09.2019 04:00



Mathematics, 26.09.2019 04:00


Computers and Technology, 26.09.2019 04:00

Biology, 26.09.2019 04:00

English, 26.09.2019 04:00

Mathematics, 26.09.2019 04:00

Mathematics, 26.09.2019 04:00