
Chemistry, 28.04.2021 19:50 loganharper992
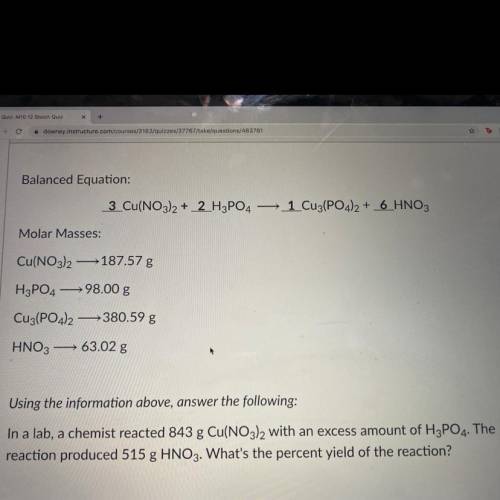
In a lab, a chemist reacted 843 g Cu(NO3)2 with an excess amount of H3PO4. The reaction produced 515 g HNO3. What's the percent yield of the reaction?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

You know the right answer?
In a lab, a chemist reacted 843 g Cu(NO3)2 with an excess amount of H3PO4. The
reaction produced 5...
Questions























