2. Lead (II) carbonate decomposes to produce lead (II) oxide and carbon dioxide:
Pbco,
→ PbO...

Chemistry, 28.04.2021 21:20 yulaarmstrong
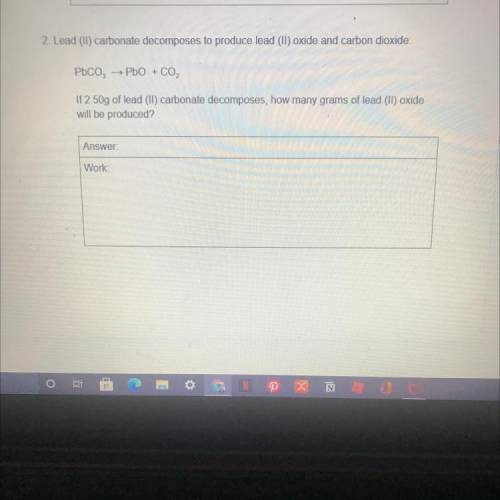
2. Lead (II) carbonate decomposes to produce lead (II) oxide and carbon dioxide:
Pbco,
→ PbO + CO2
If 2.50g of lead (II) carbonate decomposes, how many grams of lead (II) oxide
will be produced?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
Questions

History, 20.11.2020 19:50

Physics, 20.11.2020 19:50


Mathematics, 20.11.2020 19:50


History, 20.11.2020 19:50



Mathematics, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50


Mathematics, 20.11.2020 19:50


Advanced Placement (AP), 20.11.2020 19:50

French, 20.11.2020 19:50



Mathematics, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50



