
Chemistry, 28.04.2021 22:50 kodiebclay
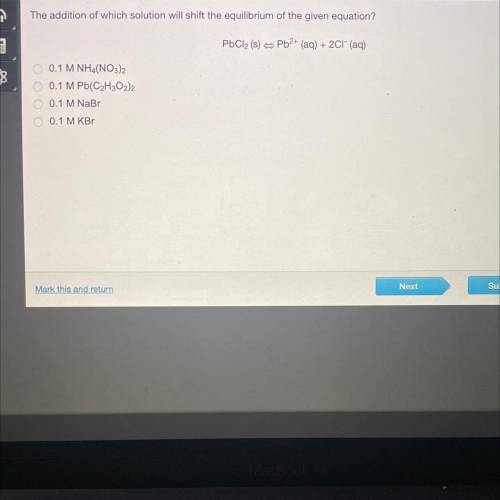
The addition of which solution will shift the equilibrium of the given equation?
PbCl2 (s) = Pb2+ (aq) + 2Cl(aq)
0 0.1 M NHA(NO3)2
0.1 M Pb(C2H302)2
0.1 M NaBr
0.1 M KB
What’s the answer?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1
You know the right answer?
The addition of which solution will shift the equilibrium of the given equation?
PbCl2 (s) = Pb2+...
Questions






Health, 25.02.2022 08:20










Mathematics, 25.02.2022 08:20



Social Studies, 25.02.2022 08:20



