
Chemistry, 29.04.2021 21:10 lauryngrace37
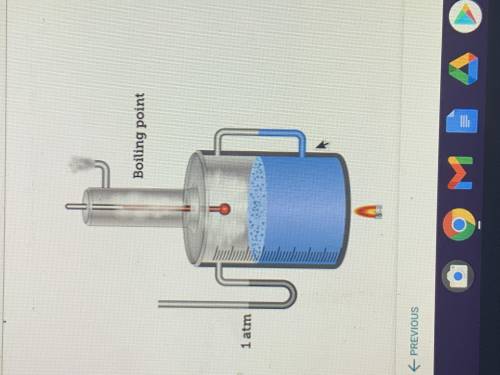
The diagram shows that the boiling point of water is 100°C At a pressure of one atm.
How could you reduce the boiling point of water in this system?
A. Increase the size of the flame
B. Decrease the volume of the water
C. Decrease the pressure to 0 atm.
D. Increase the pressure to 2 atm

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
The diagram shows that the boiling point of water is 100°C At a pressure of one atm.
How could you...
Questions




Social Studies, 12.01.2020 08:31

Mathematics, 12.01.2020 08:31



Mathematics, 12.01.2020 08:31

Mathematics, 12.01.2020 08:31



English, 12.01.2020 08:31

Social Studies, 12.01.2020 08:31


History, 12.01.2020 08:31

Mathematics, 12.01.2020 08:31

Spanish, 12.01.2020 08:31

Physics, 12.01.2020 08:31

Mathematics, 12.01.2020 08:31




