
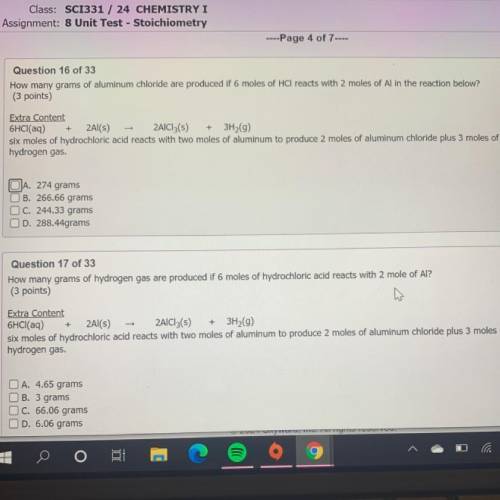
How many grams of aluminum chloride are produced if 6 moles of HCl reacts with 2 moles of Al in the reaction below?
(3 points)
Extra Content
6HCl(aq) + 2Al(s) - 2AlCl3(s) + 3H2(9)
six moles of hydrochloric acid reacts with two moles of aluminum to produce 2 moles of aluminum chloride plus 3 moles of
hydrogen gas.
JA. 274 grams
B. 266.66 grams
C. 244.33 grams
D. 288.44grams

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

You know the right answer?
How many grams of aluminum chloride are produced if 6 moles of HCl reacts with 2 moles of Al in the...
Questions

Mathematics, 30.01.2020 09:01

Chemistry, 30.01.2020 09:01


Mathematics, 30.01.2020 09:01

Chemistry, 30.01.2020 09:01

Biology, 30.01.2020 09:01

Chemistry, 30.01.2020 09:01

History, 30.01.2020 09:01



History, 30.01.2020 09:01




Mathematics, 30.01.2020 09:01



Mathematics, 30.01.2020 09:01

Mathematics, 30.01.2020 09:01



