
Chemistry, 30.04.2021 05:00 randlemccray6338
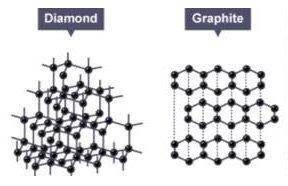
Consider the two models seen here. These models represent two different minerals. Each is composed of a lattice of carbon atoms. How do these crystals differ?
A) The two have different chemical formulas. Eliminate
B) The diamond model represents a crystal while the graphite represents a molecule.
C) Both are crystals, but the organized arrangement of the carbon atoms in each differs.
D) The graphite crystal is not pure: that is, it contains two elements while the diamond crystal is composed of carbon atoms only.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:00
3. if the dartboard below is used to model an atom, which subatomic particles would be located at z?
Answers: 2

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
Consider the two models seen here. These models represent two different minerals. Each is composed o...
Questions

Mathematics, 17.10.2021 06:50

History, 17.10.2021 06:50

Physics, 17.10.2021 06:50

Advanced Placement (AP), 17.10.2021 06:50

English, 17.10.2021 06:50

Business, 17.10.2021 06:50

Social Studies, 17.10.2021 06:50

History, 17.10.2021 06:50

English, 17.10.2021 06:50

Mathematics, 17.10.2021 06:50

English, 17.10.2021 06:50



Mathematics, 17.10.2021 06:50

Mathematics, 17.10.2021 06:50

Mathematics, 17.10.2021 06:50

English, 17.10.2021 06:50

Physics, 17.10.2021 06:50

English, 17.10.2021 06:50



