
Chemistry, 30.04.2021 15:40 fatboicroi
When 25.5 grams of a molecular substance is dissolved in 225g benzene, the solution begins to freeze at -5.05C. Calculate the molar mass of this solute (I need to understand the work, and look at the image attached for the key for benzene.
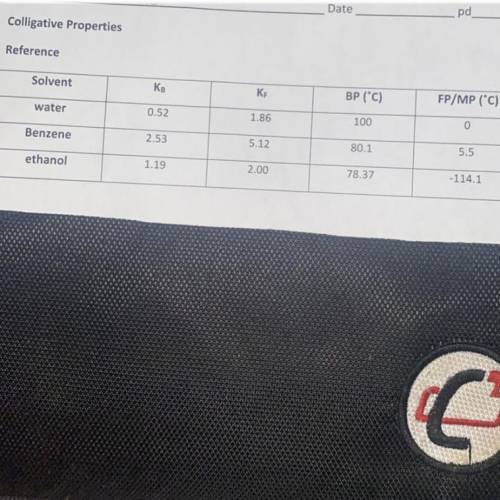

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1
You know the right answer?
When 25.5 grams of a molecular substance is dissolved in 225g benzene, the solution begins to freeze...
Questions

Mathematics, 25.09.2019 06:00

Mathematics, 25.09.2019 06:00


Mathematics, 25.09.2019 06:00


Mathematics, 25.09.2019 06:00








Mathematics, 25.09.2019 06:00


Chemistry, 25.09.2019 06:00






