
Chemistry, 30.04.2021 19:40 james22000
A mixture of nitrogen and oxygen in a 1:3 ratio has a volume of 4.00 L.
What is the volume of the nitrogen trioxide when the nitrogen and
oxygen react according to the equation while keeping pressure and temperature constant?
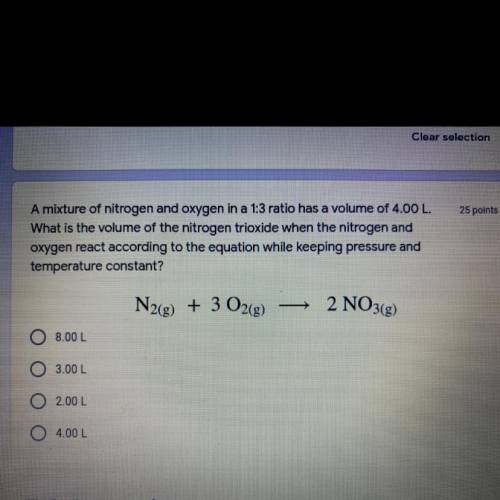

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

You know the right answer?
A mixture of nitrogen and oxygen in a 1:3 ratio has a volume of 4.00 L.
What is the volume of the...
Questions


Chemistry, 19.03.2021 19:40

Mathematics, 19.03.2021 19:40


Mathematics, 19.03.2021 19:40

Mathematics, 19.03.2021 19:40

Mathematics, 19.03.2021 19:40



Mathematics, 19.03.2021 19:40

Mathematics, 19.03.2021 19:40

Social Studies, 19.03.2021 19:40

Mathematics, 19.03.2021 19:40


Mathematics, 19.03.2021 19:40

Mathematics, 19.03.2021 19:40




Physics, 19.03.2021 19:40



