2. For the reaction
NH3 + O2 - NO + H2O
a. Balance the equation.
b. What is the...

Chemistry, 03.05.2021 01:00 aaronnnn6998
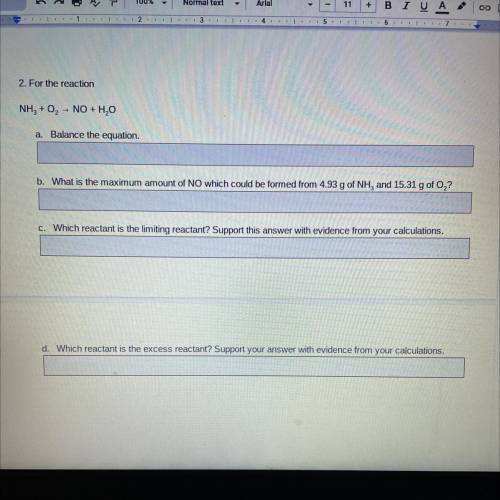
2. For the reaction
NH3 + O2 - NO + H2O
a. Balance the equation.
b. What is the maximum amount of NO which could be formed from 4.93 g of NH, and 15.31 g of O,?
C. Which reactant is the limiting reactant? Support this answer with evidence from your calculations.
d. Which reactant is the excess reactant? Support your answer with evidence from your calculations.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1

Chemistry, 23.06.2019 07:00
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1

Chemistry, 23.06.2019 09:30
What is the force of an object when it landed(sitting in the ground)
Answers: 2

Chemistry, 23.06.2019 14:00
How does electronegativity changes as we move from left to right across a period
Answers: 2
You know the right answer?
Questions




Chemistry, 02.12.2020 21:10


Social Studies, 02.12.2020 21:10

Mathematics, 02.12.2020 21:10


Mathematics, 02.12.2020 21:10

Mathematics, 02.12.2020 21:10



Mathematics, 02.12.2020 21:10

Business, 02.12.2020 21:10


English, 02.12.2020 21:10


Mathematics, 02.12.2020 21:10


Mathematics, 02.12.2020 21:10



