
Chemistry, 03.05.2021 19:00 kcutler8603
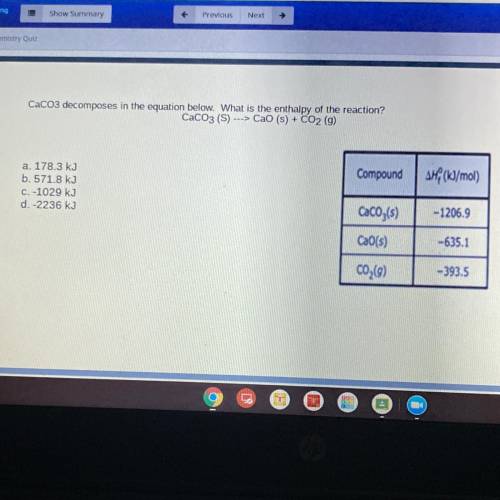
CaCO3 decomposes in the equation below. What is the enthalpy of the reaction?
CaCO3 (S) ---> Cao (s) + CO2 (g)
Compound
AH (kJ/mol)
a. 178.3 kJ
b. 571.8 kJ
C. -1029 kJ
d.-2236 kJ
CaCO3(s)
-1206.9
CaO(s)
-635.1
C02(9)
-393.5

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
CaCO3 decomposes in the equation below. What is the enthalpy of the reaction?
CaCO3 (S) ---> Ca...
Questions


Mathematics, 13.12.2019 04:31

Mathematics, 13.12.2019 04:31

History, 13.12.2019 04:31

Chemistry, 13.12.2019 04:31

Mathematics, 13.12.2019 04:31

Mathematics, 13.12.2019 04:31

Mathematics, 13.12.2019 04:31

Mathematics, 13.12.2019 04:31



Mathematics, 13.12.2019 04:31

Mathematics, 13.12.2019 04:31

Biology, 13.12.2019 04:31

History, 13.12.2019 04:31




Biology, 13.12.2019 04:31

Chemistry, 13.12.2019 04:31



