
Chemistry, 03.05.2021 19:00 johndous3698
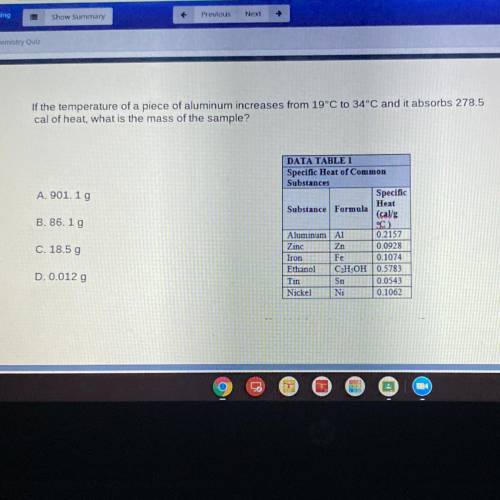
If the temperature of a piece of aluminum increases from 19°C to 34°C and it absorbs 278.5
cal of heat, what is the mass of the sample?
A. 901.19
B. 86.19
DATA TABLE 1
Specific Heat of Common
Substances
Specific
Heat
Substance Formula
(cal/g
°C
Aluminum A1 0.2157
Zinc Zn 0.0928
Iron Fe 0.1074
Ethanol C2H5OH 0.5783
Tin Sn 0.0543
Nickel Ni 0.1062
C. 18.5 g
D. 0.012 g

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1
You know the right answer?
If the temperature of a piece of aluminum increases from 19°C to 34°C and it absorbs 278.5
cal of...
Questions




Mathematics, 23.03.2021 23:50


History, 23.03.2021 23:50


Mathematics, 23.03.2021 23:50


Social Studies, 23.03.2021 23:50

Social Studies, 23.03.2021 23:50



Computers and Technology, 23.03.2021 23:50


Mathematics, 23.03.2021 23:50


Mathematics, 23.03.2021 23:50




