
Chemistry, 03.05.2021 19:00 underswap25
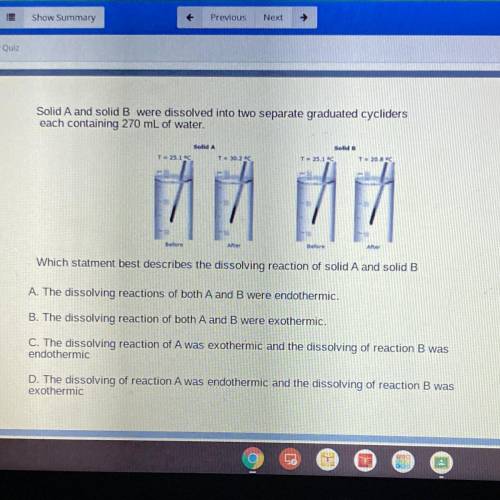
Solid A and solid B were dissolved into two separate graduated cycliders
each containing 270 mL of water.
Which statment best describes the dissolving reaction of solid A and solid B
A. The dissolving reactions of both A and B were endothermic.
B. The dissolving reaction of both A and B were exothermic.
C. The dissolving reaction of A was exothermic and the dissolving of reaction B was
endothermic
D. The dissolving of reaction A was endothermic and the dissolving of reaction B was
exothermic

Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
You know the right answer?
Solid A and solid B were dissolved into two separate graduated cycliders
each containing 270 mL of...
Questions

Mathematics, 21.08.2020 16:01

Mathematics, 21.08.2020 16:01







Mathematics, 21.08.2020 16:01

Mathematics, 21.08.2020 16:01

Mathematics, 21.08.2020 16:01









Mathematics, 21.08.2020 16:01



