
Chemistry, 03.05.2021 19:10 joshua13338
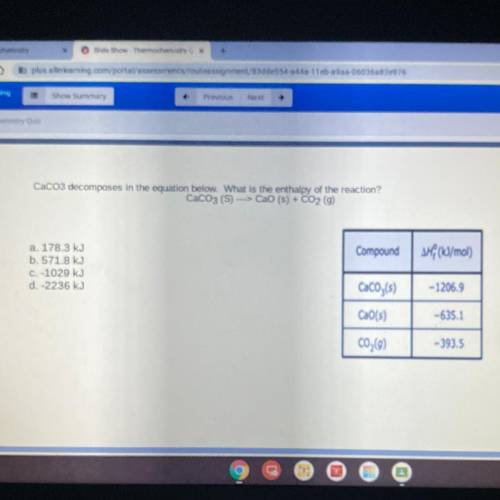
CaCo3 decomposes in the equation below. What is the enthalpy of the reaction?
CaCO3 (S) ---> CaO (s) + CO2 (g)
a. 178.3 kJ
b. 571.8 kJ
C. -1029 kJ
d. -2236 kJ

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2


You know the right answer?
CaCo3 decomposes in the equation below. What is the enthalpy of the reaction?
CaCO3 (S) ---> Ca...
Questions

Physics, 21.09.2019 12:30

History, 21.09.2019 12:30



Mathematics, 21.09.2019 12:30

Mathematics, 21.09.2019 12:30


Social Studies, 21.09.2019 12:30

Mathematics, 21.09.2019 12:30


Social Studies, 21.09.2019 12:30



History, 21.09.2019 12:30

History, 21.09.2019 12:30



Mathematics, 21.09.2019 12:30


French, 21.09.2019 12:30



