
Chemistry, 03.05.2021 19:20 ayowazzzgood
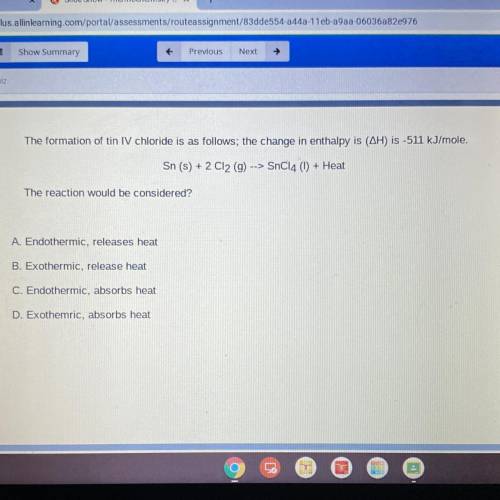
The formation of tin IV chloride is as follows; the change in enthalpy is (AH) is -511 kJ/mole.
Sn (s) + 2 C12 (9) --> SnC14 (1) + Heat
The reaction would be considered?
A. Endothermic, releases heat
B. Exothermic, release heat
C. Endothermic, absorbs heat
D. Exothemric, absorbs heat

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3


Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1
You know the right answer?
The formation of tin IV chloride is as follows; the change in enthalpy is (AH) is -511 kJ/mole.
Sn...
Questions

English, 26.10.2019 20:43



Business, 26.10.2019 20:43

Mathematics, 26.10.2019 20:43



Mathematics, 26.10.2019 20:43

Chemistry, 26.10.2019 20:43


Mathematics, 26.10.2019 20:43

Mathematics, 26.10.2019 20:43


Geography, 26.10.2019 20:43


Geography, 26.10.2019 20:43

English, 26.10.2019 20:43



History, 26.10.2019 20:43



