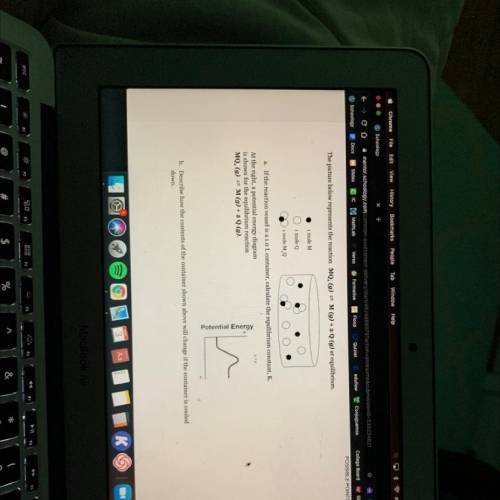
The picture below represents the reaction MQ, (g) = M(g) + 2 Q (g) at equilibrium.
1 mole M
...

Chemistry, 03.05.2021 20:10 hihihi129473838
The picture below represents the reaction MQ, (g) = M(g) + 2 Q (g) at equilibrium.
1 mole M
i mole Q
1 mole MQ
a. If the reaction vessel is a 1.0 L container, calculate the equilibrium constant, K.
At the right, a potential energy diagram
is shown for the equilibrium reaction
MQ. (g) = M(g) + 2 Q (9).
Potential Energy
b. Describe how the contents of the container shown above will change if the container is cooled
down.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3
You know the right answer?
Questions













Mathematics, 06.06.2020 01:02





History, 06.06.2020 01:02

Mathematics, 06.06.2020 01:02



