
Chemistry, 03.05.2021 21:10 estefaniapenalo
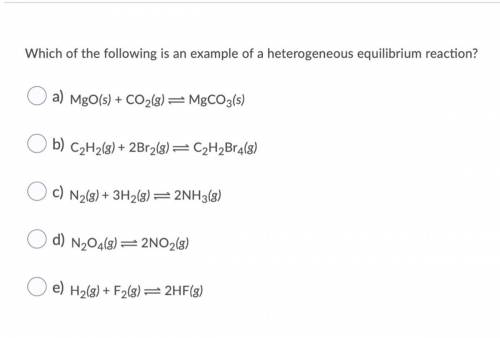
Which of the following is an example of a heterogeneous equilibrium reaction? O a) MgO(s) + CO2(g) = MgCO3(s) O b) C2H2(g) + 2Br2(g) = C2H2Br4(8) O c) N2(g) + 3H2(g) = 2NH3(8) O d) N204(8) =2NO2(8) O e) H2(g) + F2(8)=2HF(3)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1


Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
Which of the following is an example of a heterogeneous equilibrium reaction? O a) MgO(s) + CO2(g) =...
Questions


Mathematics, 11.11.2020 06:00


English, 11.11.2020 06:00



Mathematics, 11.11.2020 06:00


History, 11.11.2020 06:00

Arts, 11.11.2020 06:00

Biology, 11.11.2020 06:00


History, 11.11.2020 06:00


History, 11.11.2020 06:00




Mathematics, 11.11.2020 06:00

Biology, 11.11.2020 06:00



