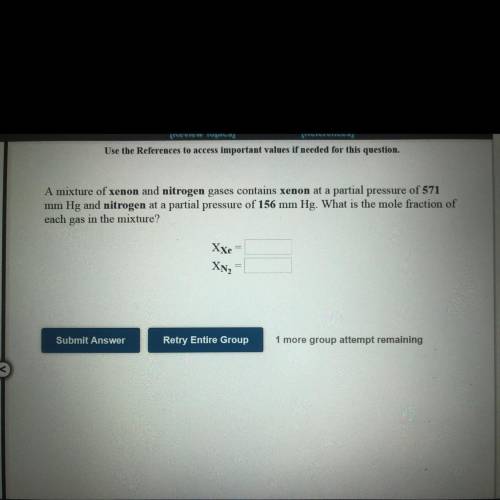
A mixture of xenon and nitrogen gases contains xenon at a partial pressure
of 571
mm Hg and...

Chemistry, 04.05.2021 06:10 chriscook4471
A mixture of xenon and nitrogen gases contains xenon at a partial pressure
of 571
mm Hg and nitrogen at a partial pressure of 156 mm Hg. What is the mole fraction of
each gas in the mixture?
Please look at the picture above
XXe=?
XN2=?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
Questions



Social Studies, 06.07.2019 10:00





Chemistry, 06.07.2019 10:00


History, 06.07.2019 10:00


Mathematics, 06.07.2019 10:00

English, 06.07.2019 10:00



Mathematics, 06.07.2019 10:00




English, 06.07.2019 10:00



