
Using the amount of each element you calculated in part A, calculate the ratios of the elements to each other to
determine the empirical formula.
Select the correct answer from each drop-down menu to complete the empirical formula.
С
- Н
0
I HAVE THE ANSWER THIS IS FOR WHO NEEDS IT.
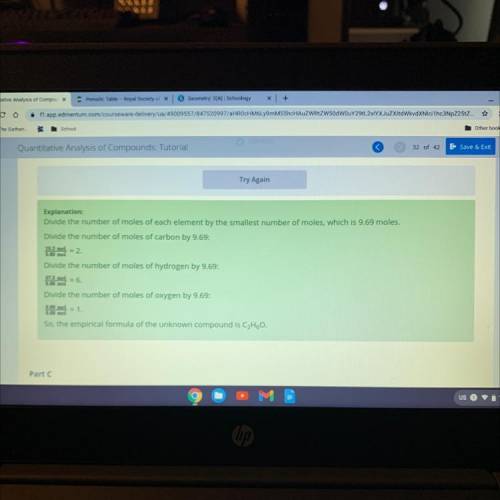

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1


Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
Using the amount of each element you calculated in part A, calculate the ratios of the elements to e...
Questions

History, 07.05.2020 03:57


Mathematics, 07.05.2020 03:57





Mathematics, 07.05.2020 03:57


Mathematics, 07.05.2020 03:57

Biology, 07.05.2020 03:57




Mathematics, 07.05.2020 03:57

Mathematics, 07.05.2020 03:57


Computers and Technology, 07.05.2020 03:57




