
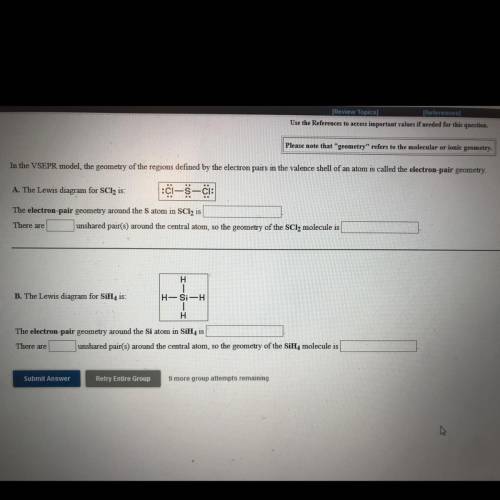
Use the References to access important values if needed for this question.
Please note that "geometry" refers to the molecular or ionic geometry.
In the VSEPR model, the geometry of the regions defined by the electron pairs in the valence shell of an atom is called the electron-pair geometry.
A. The Lewis diagram for SCl2 is: C-5-c:
The electron-pair geometry around the Satom in SCl2 is
There are
unshared pair(s) around the central atom, so the geometry of the SCl2 molecule is
H
B. The Lewis diagram for SiH, is:
H-Si-H
H
The electron-pair geometry around the Si atom in SiH, is
There are unshared pair(s) around the central atom, so the geometry of the SiH, molecule is
Submit Answer
Retry Entire Group
9 more group attempts remaining

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is mostly likely why many scientists reject the cold fusion theory
Answers: 1

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3
You know the right answer?
Use the References to access important values if needed for this question.
Please note that "geome...
Questions

History, 08.12.2020 22:50

Mathematics, 08.12.2020 22:50

Mathematics, 08.12.2020 22:50

Chemistry, 08.12.2020 22:50

Mathematics, 08.12.2020 22:50

Chemistry, 08.12.2020 22:50

History, 08.12.2020 22:50

Mathematics, 08.12.2020 22:50








History, 08.12.2020 22:50

Mathematics, 08.12.2020 22:50


Mathematics, 08.12.2020 22:50




