
Chemistry, 05.05.2021 02:10 peachijmin
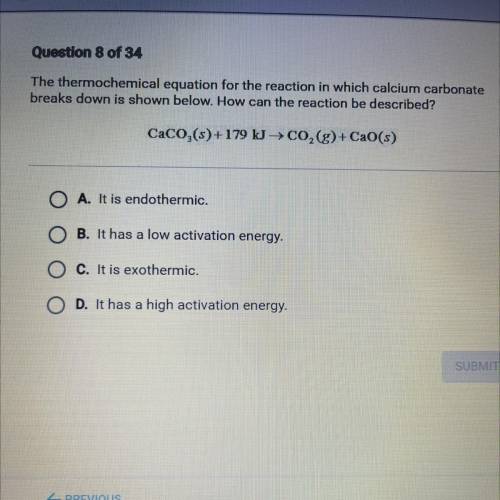
The thermochemical equation for the reaction in which calcium carbonate
breaks down is shown below. How can the reaction be described?
CaCO,(s)+179 kg CO,(g)+ CaO(s)
A. It is endothermic.
B. It has a low activation energy.
C. It is exothermic.
D. It has a high activation energy.
NO LINKS

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2


Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
The thermochemical equation for the reaction in which calcium carbonate
breaks down is shown below...
Questions

Mathematics, 07.10.2020 14:01

Advanced Placement (AP), 07.10.2020 14:01

Mathematics, 07.10.2020 14:01

Chemistry, 07.10.2020 14:01



Mathematics, 07.10.2020 14:01




Mathematics, 07.10.2020 14:01


Advanced Placement (AP), 07.10.2020 14:01


Social Studies, 07.10.2020 14:01



Social Studies, 07.10.2020 14:01




