

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
4. Balance the following equation. Then determine the number of mols of Nitrogen formed given the va...
Questions

Mathematics, 18.09.2019 07:30

Chemistry, 18.09.2019 07:30



English, 18.09.2019 07:30


Health, 18.09.2019 07:30

Mathematics, 18.09.2019 07:30


Mathematics, 18.09.2019 07:30

Mathematics, 18.09.2019 07:30

Mathematics, 18.09.2019 07:30

Biology, 18.09.2019 07:30




History, 18.09.2019 07:30



History, 18.09.2019 07:30

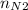 = 4 × 2 ÷ 4 = 2
= 4 × 2 ÷ 4 = 2 

