
Which conclusion is best supported by the data shown in the graph above?
A. As a solution becomes more acidic or basic, the conductivity of the solution decreases.
B. As the pH of a solution decreases, the conductivity of the solution increases.
C. As a solution becomes more acidic or more basic, the conductivity of the solution increases.
D. As the pH of a solution increases, the conductivity of the solution decreases.
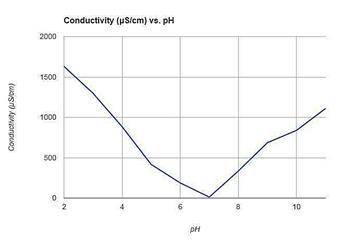

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
Which conclusion is best supported by the data shown in the graph above?
A. As a solution becomes...
Questions

Mathematics, 11.01.2022 04:00



Mathematics, 11.01.2022 04:00




English, 11.01.2022 04:10

History, 11.01.2022 04:10





Law, 11.01.2022 04:10





Physics, 11.01.2022 04:10



