
Chemistry, 06.05.2021 05:30 crodriguez87
Given the following thermochemical equation, how much energy is given off when 27.4 g of N2 reacts?
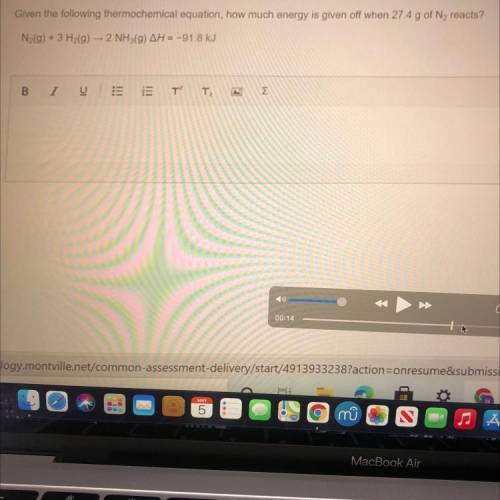

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
Given the following thermochemical equation, how much energy is given off when 27.4 g of N2 reacts?...
Questions




History, 09.12.2020 22:50




Mathematics, 09.12.2020 22:50

History, 09.12.2020 22:50






Mathematics, 09.12.2020 22:50

Business, 09.12.2020 22:50

Mathematics, 09.12.2020 22:50


Mathematics, 09.12.2020 22:50



