
Chemistry, 06.05.2021 05:40 aaliyahrice02
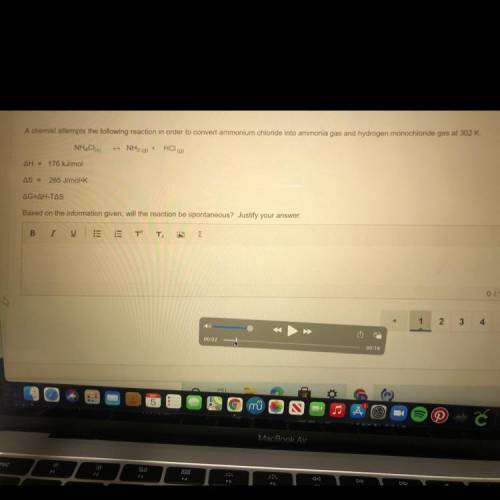
A chemist attempts the following reaction in order to convert ammonium chloride into ammonia gas and hydrogen monochloride gas at 302 K. Based on the information given, will the reaction be spontaneous? Justify your answer.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:40
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b.zinc7.14,c.copper 8.92,d.lead 11.34
Answers: 2

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3
You know the right answer?
A chemist attempts the following reaction in order to convert ammonium chloride into ammonia gas and...
Questions



Mathematics, 25.05.2021 17:30



Chemistry, 25.05.2021 17:30

Mathematics, 25.05.2021 17:30

Arts, 25.05.2021 17:30




Mathematics, 25.05.2021 17:30

Business, 25.05.2021 17:30



Mathematics, 25.05.2021 17:30



Arts, 25.05.2021 17:30

Mathematics, 25.05.2021 17:30



