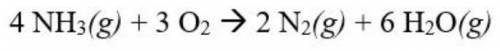Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
Ammonia gas (NH3) combines with oxygen gas (O2) to form diatomic nitrogen gas and water vapor. If 4....
Questions

History, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

History, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Geography, 18.09.2020 22:01

Health, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Geography, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01