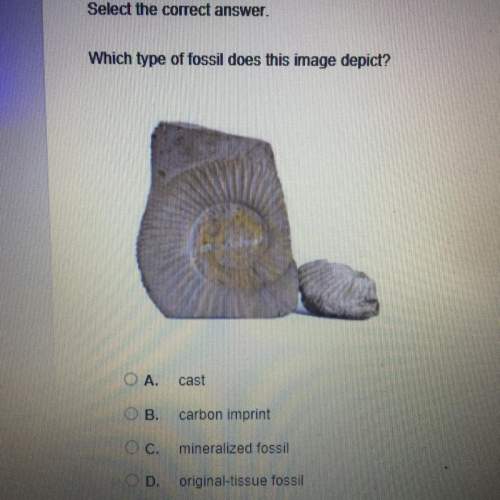
Chemistry, 06.05.2021 22:10 obliviousho2018
A beaker of solution contains 5.86 L of 13.72 M solution. What will be the volume of the solution after it is diluted to 5.38 M? Please help❤️

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which type of stress results when two plates push against one another? a. compression b. tension c. force d. shear
Answers: 1

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1
You know the right answer?
A beaker of solution contains 5.86 L of 13.72 M solution. What will be the volume of
the solution...
Questions

Mathematics, 02.10.2020 15:01

English, 02.10.2020 15:01


Mathematics, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01


Mathematics, 02.10.2020 15:01




History, 02.10.2020 15:01


Mathematics, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01

Arts, 02.10.2020 15:01

History, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01

History, 02.10.2020 15:01

English, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01