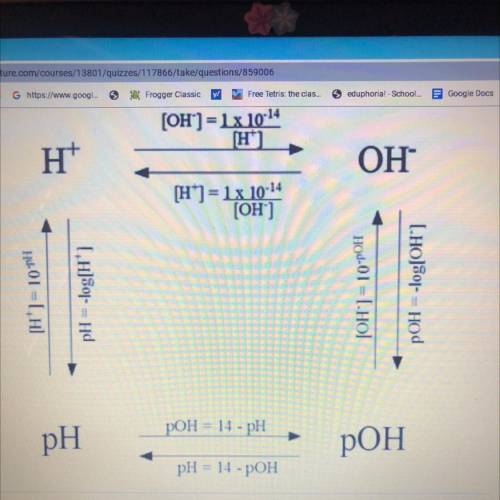
What is the pH of an aqueous solution with a hydrogen ion concentration of 1.8x10^-4M?
...


Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 21.06.2019 18:40
What kind of ion is contained in salts that produce an acidic solution? a positive ion that attracts a proton from water a positive ion that releases a proton to water a negative ion that attracts a proton from water a negative ion that releases a proton to water
Answers: 1

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
Questions

Health, 07.05.2021 14:00

Mathematics, 07.05.2021 14:00

English, 07.05.2021 14:00

Social Studies, 07.05.2021 14:00


Computers and Technology, 07.05.2021 14:00


Chemistry, 07.05.2021 14:00

Mathematics, 07.05.2021 14:00

English, 07.05.2021 14:00

Mathematics, 07.05.2021 14:00



Physics, 07.05.2021 14:00

History, 07.05.2021 14:00


Advanced Placement (AP), 07.05.2021 14:00

Mathematics, 07.05.2021 14:00


Chemistry, 07.05.2021 14:00