
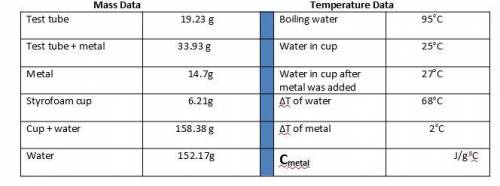
1. Calculate the heat gained by the water (lost by the metal) in the calorimeter using the equation in the introduction.
Metal A
Q = mc(ΔT)
Qwater = -Qmetal
Heat gained = Mass of x Specific heat of x Change in temperature
by the water water (g) water (4.184 J/goC) (ΔT)
The specific heat of the metal can now be calculated:
Specific heat = Heat gained by the water
of metal (c) Mass of metal (g) x ΔT of metal (oC)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 23.06.2019 10:20
Determine the mass of the object below with accuracy and to the correct degree of precision. a. 324.2 g b. 324 g c. 324.30 g d. 324.25 g
Answers: 3

Chemistry, 23.06.2019 14:00
Which is not true regarding reaction rates? (2 points) catalysts are not used up in the reaction. catalysts speed up reactions by lowering the activation energy. reaction rates decrease as the concentration of reactants decrease. during reactions, concentrations of all reactants decrease at the same rate.
Answers: 1
You know the right answer?
1. Calculate the heat gained by the water (lost by the metal) in the calorimeter using the equation...
Questions


English, 23.05.2020 22:04

Mathematics, 23.05.2020 22:04

Advanced Placement (AP), 23.05.2020 22:04

Mathematics, 23.05.2020 22:04


Biology, 23.05.2020 22:04

Mathematics, 23.05.2020 22:04





History, 23.05.2020 22:04

Geography, 23.05.2020 22:04

Mathematics, 23.05.2020 22:04

Social Studies, 23.05.2020 22:04

History, 23.05.2020 22:04





