
Chemistry, 07.05.2021 03:20 Honeyswish7730
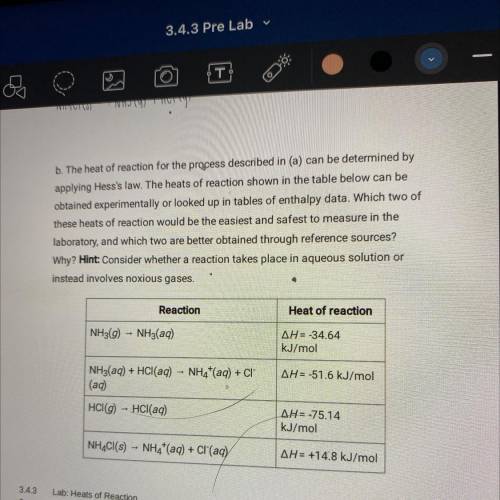
B. The heat of reaction for the process described in (a) can be determined by
applying Hess's law. The heats of reaction shown in the table below can be
obtained experimentally or looked up in tables of enthalpy data. Which two of
these heats of reaction would be the easiest and safest to measure in the
laboratory, and which two are better obtained through reference sources?
Why? Hint: Consider whether a reaction takes place in aqueous solution or
instead involves noxious gases.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1

You know the right answer?
B. The heat of reaction for the process described in (a) can be determined by
applying Hess's law....
Questions



Biology, 06.10.2019 19:50


Mathematics, 06.10.2019 19:50



Mathematics, 06.10.2019 19:50



English, 06.10.2019 19:50



Mathematics, 06.10.2019 19:50





Biology, 06.10.2019 20:00

Mathematics, 06.10.2019 20:00



