
Chemistry, 07.05.2021 03:40 keonburkes9
The bond dissociation energy to break 8 C-H bond(s) in 1 mole of hexane (CH₃CH₂CH₂CH₂CH₂CH₃) molecules is
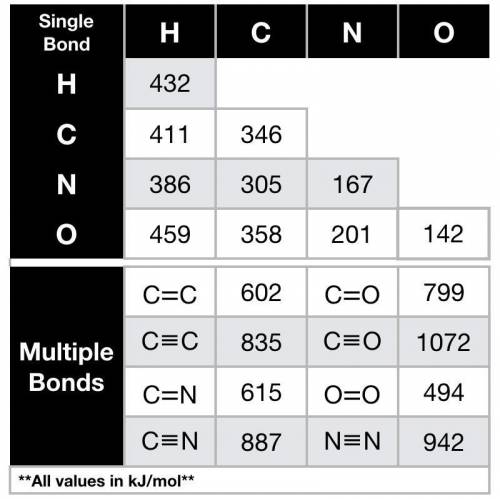

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2


Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
The bond dissociation energy to break 8 C-H bond(s) in 1 mole of hexane (CH₃CH₂CH₂CH₂CH₂CH₃) molecul...
Questions


Mathematics, 27.01.2021 17:40

Mathematics, 27.01.2021 17:40

Mathematics, 27.01.2021 17:40

Chemistry, 27.01.2021 17:40

SAT, 27.01.2021 17:40

Mathematics, 27.01.2021 17:40

Chemistry, 27.01.2021 17:40

Mathematics, 27.01.2021 17:40




Chemistry, 27.01.2021 17:40

Arts, 27.01.2021 17:40



Mathematics, 27.01.2021 17:40

Mathematics, 27.01.2021 17:40

History, 27.01.2021 17:40

Mathematics, 27.01.2021 17:40



