
Chemistry, 07.05.2021 16:30 nicolasliberat
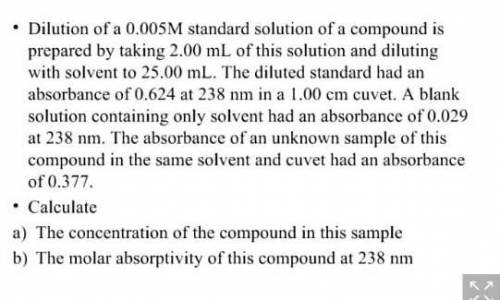
Dilution of a 0.005M standard solution of a compound is prepared by taking 2.00 mL of this solution and diluting with solvent to 25.00 mL. The diluted standard had an absorbance of 0.624 at 238 nm in a 1.00 cm cuvet. A blank solution containing only solvent had an absorbance of 0.029 at 238 nm. The absorbance of an unknown sample of this compound in the same solvent and cuvet had an absorbance of 0.377. • Calculate a) The concentration of the compound in this sample b) The molar absorptivity of this compound at 238 nm

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1



Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
You know the right answer?
Dilution of a 0.005M standard solution of a compound is prepared by taking 2.00 mL of this solution...
Questions

Mathematics, 24.11.2020 01:00


Biology, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00


History, 24.11.2020 01:00


Mathematics, 24.11.2020 01:00




Biology, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00



Mathematics, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00

Chemistry, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00



