
Chemistry, 07.05.2021 22:30 aallyssabrown0120
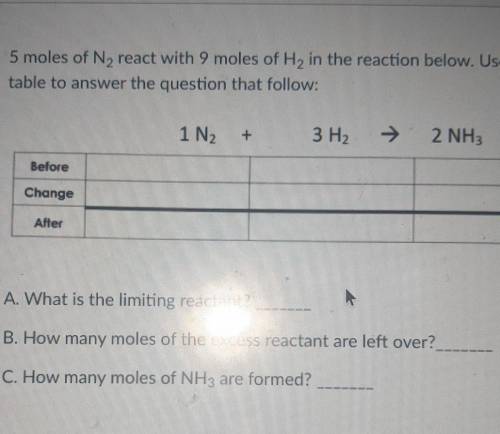
5 moles of N2 react with 9 moles of H2 in the reaction below. Use the BCA table to answer the question that follow: 1N2+3H2=2NH3
A. What is the limiting reactant?
B. How many moles of the excess reactant are left over?
C. How many moles of NH3 are formed?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 06:00
Give one example of a pure (exact) number and of an estimated (measured) number.
Answers: 2
You know the right answer?
5 moles of N2 react with 9 moles of H2 in the reaction below. Use the BCA table to answer the questi...
Questions















Social Studies, 20.12.2019 05:31




History, 20.12.2019 05:31




