
Chemistry, 08.05.2021 01:00 mckenziealexander
When sodium is placed in water, the following chemical reaction occurs. 2Na(s) + 2H2O (l) —> 2NaOH (aq) + H2 (g). If a teacher uses a 0.500 gram sample of sodium metal for a demonstration with excess water, how many liters of hydrogen gas would be produced at STP?
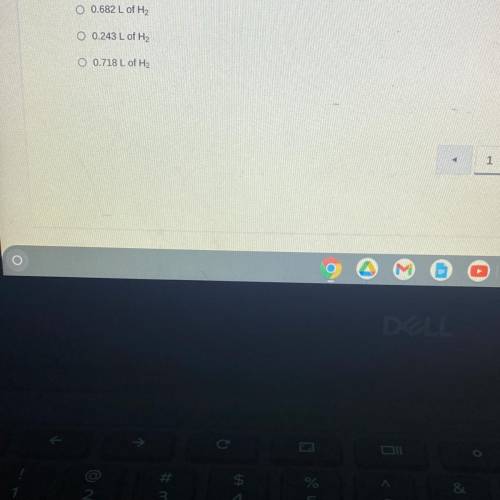

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 00:30
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
When sodium is placed in water, the following chemical reaction occurs. 2Na(s) + 2H2O (l) —> 2NaO...
Questions

Social Studies, 04.07.2019 14:10







Mathematics, 04.07.2019 14:10



Spanish, 04.07.2019 14:10


History, 04.07.2019 14:20


Business, 04.07.2019 14:20

English, 04.07.2019 14:20

Health, 04.07.2019 14:20

Physics, 04.07.2019 14:20


Business, 04.07.2019 14:20



