
Chemistry, 08.05.2021 03:50 sammyraegarrett
Please Help Weak Acid Base Problems!!!
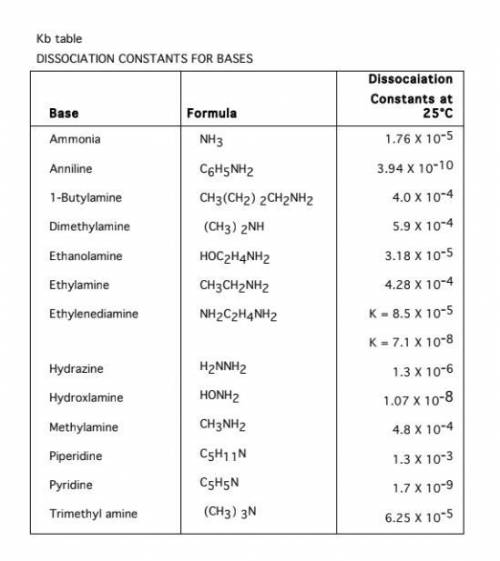
1. Calculate the pH of a 0.100 M NH3 solution Kb = 1.8 x 10^-5
Calculate the pH of a 0.100 M NH3 solution Kb = 1.8 x 10^-5
Initial:
Change:
at Equilibrium:
2. Calculate the Kb from species concentrations
Calculate the Kb for methylamine, CH3NH2, if the pOH of a 0.0100 M CH3NH2 solution is 7.66
Initial:
Change:
at Equilibrium:
3. Calculation of species concentrations from Kb
Calculate the pH of a 0.100 M C5H5N Kb == 1.3 x 10^-3
Initial:
Change:
at Equilibrium:

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

You know the right answer?
Please Help Weak Acid Base Problems!!!
1. Calculate the pH of a 0.100 M NH3 solution Kb = 1.8 x 10...
Questions



Mathematics, 19.05.2021 17:30





Mathematics, 19.05.2021 17:30

Mathematics, 19.05.2021 17:30

Mathematics, 19.05.2021 17:30

Mathematics, 19.05.2021 17:30

Spanish, 19.05.2021 17:30



Mathematics, 19.05.2021 17:30



English, 19.05.2021 17:30

Mathematics, 19.05.2021 17:30

Mathematics, 19.05.2021 17:30



