Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1


Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1
You know the right answer?
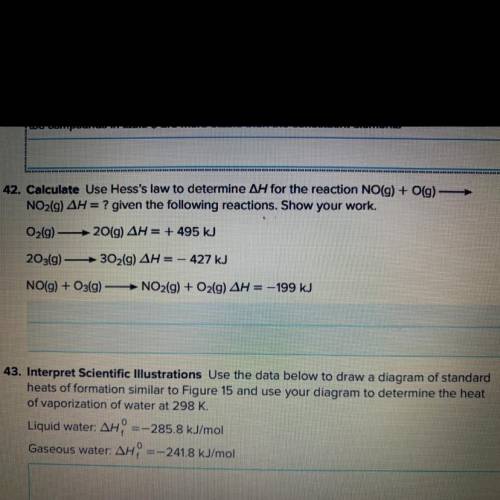
Calculate, use hess’s law to determine /\H for the reaction NO(g) + O(g)—> NO2(g) /\H=? given the...
Questions


Biology, 30.06.2019 02:00



Arts, 30.06.2019 02:00

Mathematics, 30.06.2019 02:00


Arts, 30.06.2019 02:00

Chemistry, 30.06.2019 02:00


Physics, 30.06.2019 02:00






Arts, 30.06.2019 02:00