
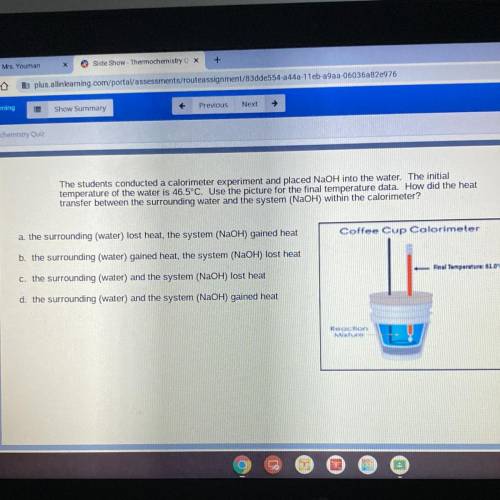
The students conducted a calorimeter experiment and placed NaOH into the water. The initial
temperature of the water is 46.5°C. Use the picture for the final temperature data. How did the heat
transfer between the surrounding water and the system (NaOH) within the calorimeter?
a. the surrounding (water) lost heat, the system (NaOH) gained heat
Coffee Cup Calorimeter
b. the surrounding (water) gained heat, the system (NaOH) lost heat
Final Temperature61.0°C
c. the surrounding (water) and the system (NaOH) lost heat
d. the surrounding (water) and the system (NaOH) gained heat
Reaction
Mixture

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
In the analysis of hair and fiber samples, which does a compound comparison microscope allow for that a conventional compound microscope does not? a. simultaneous observation b. polarization c. fluorescence d. higher magnification
Answers: 2

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1
You know the right answer?
The students conducted a calorimeter experiment and placed NaOH into the water. The initial
temper...
Questions


Mathematics, 14.06.2021 06:30






Geography, 14.06.2021 06:30

Physics, 14.06.2021 06:30


English, 14.06.2021 06:30

Business, 14.06.2021 06:30

Computers and Technology, 14.06.2021 06:30

Mathematics, 14.06.2021 06:30



Mathematics, 14.06.2021 06:30

Business, 14.06.2021 06:30


Mathematics, 14.06.2021 06:30



